Death Adders (Genus Acanthophis): An overview, including descriptions of FIVE new species and ONE subspecies.
ORIGINALLY Published in Monitor 9 (2) April 1998. Pages 20-41.
Raymond Hoser (1998 address),
41 Village Avenue, Doncaster, Victoria,
3108, Australia.
Phone: +61 3 98123322 E-mail:see end of paper
Click here for a more recent paper naming further species and subspecies of Acanthophis.![]()
INTRODUCTION
 Death
Adders (Genus Acanthophis) are found in most parts of Australia,
New Guinea and adjacent islands. They are unusual among the elapids in
that they have evolved to become viperine in appearance and habit. All
species are characterised by a broad somewhat flattened, triangular head,
short stout body and a thin rat-like body ending in a
Death
Adders (Genus Acanthophis) are found in most parts of Australia,
New Guinea and adjacent islands. They are unusual among the elapids in
that they have evolved to become viperine in appearance and habit. All
species are characterised by a broad somewhat flattened, triangular head,
short stout body and a thin rat-like body ending in a  curved
spine. The spine and the presence of subocular scales separates Acanthophis
from all other Australasian elapids.
curved
spine. The spine and the presence of subocular scales separates Acanthophis
from all other Australasian elapids.
A detailed summary of these snakes, in Australia as of 1995, including biology, ecology and other relevant information, as well as a substantial bibliography was published by Hoser (1995). That paper can be downloaded from the world-wide-web at http://www.smuggled.com/adder1.htm and is strongly recommended to any reader seeking a detailed background of this genus.
There has been substantial confusion and misinformation regarding the taxonomy of species within the genus Acanthophis, particularly for those in northern Australia and Islands to the north, including New Guinea. This confusion has been caused by several factors including the fact that a number of well-known authors have made taxonomic judgments without inspecting animals previously described and/or relatively scant knowledge of the snakes in question. The purpose of this paper is to review the current taxonomy and formally describe known species and subspecies which to date have not carried scientific names.
 The basis of this review comes from studies of these snakes over
a period in excess of 20 years, a review of published literature, (not
all of which is cited here or in Hoser 1995), discussions with many private
and professional herpetologists and keepers as well as the inspection of
a substantial number of live snakes and others preserved in collections.
The author has observed live specimens of all species and subspecies known
from Australia (listed below) and some live animals from New Guinea when
on tour in the United States in 1993.
The basis of this review comes from studies of these snakes over
a period in excess of 20 years, a review of published literature, (not
all of which is cited here or in Hoser 1995), discussions with many private
and professional herpetologists and keepers as well as the inspection of
a substantial number of live snakes and others preserved in collections.
The author has observed live specimens of all species and subspecies known
from Australia (listed below) and some live animals from New Guinea when
on tour in the United States in 1993.
Taxonomically, Death Adders present substantial problems in species identification due to the high degree of variability within each species, including within any single local population and the fact that many identifying characteristics sometimes used to separate species are shared to varying degrees by multiple species. Major character differences, such as base colour (i.e. red versus grey), may be affected by as little as one single gene (allele), (Hoser, 1985), clearly indicating that use of such a character on it's own to separate species would be hazardous to say the least.
Previous works that cover Acanthophis taxonomy, include: Boulenger, (1898), Cogger (1983, 1992), Loveridge, (1948), Macleay, (1877), McDowall (1984), O'Shea (1996), Ramsay, (1877), Shaw and Nodder, (1802) Storr (1981) and Wells and Wellington (1983, 1985a, 1985b). Cogger (1992) and Ehmann (1992), both indicate undescribed forms of Acanthophis, or at least species other than the most commonly recognised trio, namely, A. antarcticus, A. pyrrhus and A. praelongus.
The use of venom properties to aid in distinguishing between forms and species has increased in recent years. Such has not been attempted by this author although venom supplied by this author has been used by others including Sheumack, et. al.. Papers dealing with venom properties of Acanthophis include: Fairley (1929), Kim and Tamiya (1981), Sheumack, Howden and Spence (1979), Stettler (1985), and van Woerkom (1985).
Keys that differentiate different species of Acanthophis have not been presented here. All keys seen by this author for the genus Acathophis appear to break down with susbstantial regularity due to veriablity within each species, even though a number of species divisons are widely acknowledged.
The keys of both Cogger (1992) and Storr (1981) for the species A. antarcticus, A. praelongus and A. pyrrhus regularly break down when used against their divisions of Acanthophis into those three forms (breakdown of keys is generally between A. antarcticus and A. praelongus). However their keys do indicate trends in differences between the different forms. Keys have tended to rely on external characteristics such as colour patterns and rugosity, rather than head and body scalation, due to the variability of the latter within a single species, and corresponding relative uniformity of the trait within the genus.
For the purposes of this paper, a species of Acanthophis is defined as a population that appears to be different from others in physical characteristics, including those known to occur in nearby areas, but for which there is presently no evidence of gene flow between the populations. This definition does not take into account relationships between snakes in a captive situation. For example in 1996 a captive male Acanthophis (similar to and possibly A. hawkei) from near Camooweal, Queensland, mated with a female Hayes Creek, Northern Territory A. lancasteri to produce 31 healthy offspring in 1997, most of which were still alive and well in April 1998 (there were an additional five stillborn and no unfertilised ova). One of these snakes that were bred by Rob Valentic is depicted on the front cover of this magazine. Colouration of young tends to be intermediate between those of the parents, although colouration of offspring was not consistent.
Hoser (1989) published a photo of a captive male A. pyrrhus attempting to mate with an A. antarcticus. I have also observed both captive A. praelongus (from Queensland) and A. lancasteri (from Western Australia) attempting to mate with A. antarcticus (from New South Wales), while the captive male A. antarcticus pictured on the back cover of Hoser, (1989), was observed attempting to copulate with a female A. pyrrhus (armstrongi).
As of 1998, there have been no records of sympatry in the wild between different species of Acanthophis, a point noted by McDowall (1984). Although there is no dispute in the fact that all Acanthophis are closely related, it is uncertain if given similarities between different species reflect immediate relationships or are instead due to convergence in evolution to cope with localised conditions.
For each species listed below, I have made comments relating to the taxonomy and present understanding of each, including with reference to comments made by earlier authors. Previously unpublished information for some species of Acanthophis is given where appropriate. The listing of species and subspecies given in this paper completes that currently known for this genus. It is likely that further species and/or subspecies may later be recognised, particularly for island populations, many of which are suspected as differing from those of adjacent "mainland" populations. There has been no investigation into Acanthophis from the large islands west and north-west of New Guinea, such as Ceram or Halmahera Island and none have been sighted by this author. Attempts to get hold of specimens, photos and other material have so far been unsuccessful.
Due to reliance on head scalation as a diagnostic feature for some species of Acanthophis listed below, a drawing of the head scalation of a West Head (about 30 km north of Sydney, NSW) A. antarcticus from p. 18, (Hoser 1989), is reproduced here to assist readers in familiarising themselves with the relevant scales and positions.
As of the publication of this paper, there are now 11 species of Acanthophis now recognised, along with two additional subspecies. Four of the 11 species are known only from the island of New Guinea (including offshore islands), while the remaining 7 are known only from continental Australia and offshore islands.
 SPECIES
AND SUBSPECIES OF ACANTHOPHIS NOW RECOGNISED
SPECIES
AND SUBSPECIES OF ACANTHOPHIS NOW RECOGNISED
Acanthophis antarcticus antarcticus (Shaw and Nodder, 1802)
Acanthophis antarcticus schistos Wells and Wellington, 1985
Acanthophis barnetti sp. nov. (this paper).
Acanthophis crotalusei sp. nov. (this paper).
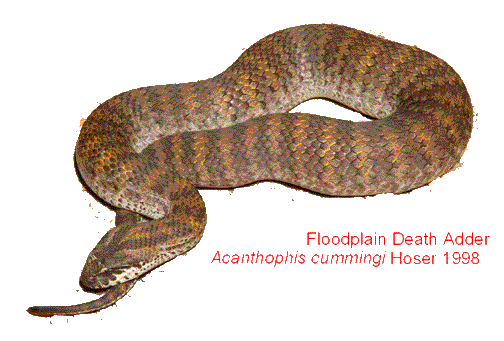
Acanthophis cummingi sp. nov. (this paper).
Acanthophis hawkei Wells and Wellington 1985
Acanthophis laevis Macleay, 1877
Acanthophis lancasteri lancasteri Wells and Wellington 1985
Acanthophis lancasteri bottomi subsp. nov. (this paper).
Acanthophis praelongus Ramsay, 1877
Acanthophis pyrrhus Boulenger, 1898
Acanthophis pyrrhus armstrongi Wells and Wellington, 1985
Acanthophis rugosus Loveridge, 1948
Acanthophis wellsei sp. nov. (this paper.)
Acanthophis woolfi sp. nov. (this paper.)
(Total of 12 species and three additional subspecies)
Acanthophis antarcticus antarcticus (Shaw and Nodder, 1802)
Type data lost, however modern authors have assumed that the "Sydney" Death Adders are of this species, treating it as the "typical" form. Cogger (1983) made the following species names junior synonyms, palpebrosa, cerastinus, brownii, ambigua, acantophis, sorda and aculeata. All had missing type data and/or specimens. Known distribution of A. antarcticus is southern Australia (except coldest parts), and the east coast and adjacent areas, including much of inland Queensland. There is a gap in the known distribution in the region of the SA, Vic, NSW border area.
There are no reliable modern records for the state of Victoria.,
(Coventry 1997). The National Museum of Victoria has NO  authentic
locality records for the species (of any date). There are several old specimens
labelled "Murray River Victoria", but no precise locality data
(Coventry 1997).
authentic
locality records for the species (of any date). There are several old specimens
labelled "Murray River Victoria", but no precise locality data
(Coventry 1997).
An alleged sighting record of Peter Menkhorst dated 1994 (Menkhorst 1994) from Wallpolla Island, Murray River, is not deemed reliable as it was not made by a trained herpetologist or corroborated by a photograph or specimen. Contrary to popular belief there is no spinifex (Triodia sp.) on the island (Coventry 1997). The diary of Gerard Krefft has an 1856 entry of a drawing of a head and tail of this species from Lake Boga, which is about 15 km south-east of Swan Hill, in Victoria. Coventry and Robertson (1991) concluded that the species no longer occurs there due to habitat destruction for farming.
Specimens of Acanthophis from Mount Isa, Cloncurry, Dutchess and Dajarra, Queensland which themselves vary significantly in appearance, and are herein referred to as A. woolfi should be investigated further. Externally, they appear to be intermediate between A. hawkei (see p. 34 this journal for photos of a juvenile and adult A. woolfi) and A. antarcticus from more southern areas in general characteristics; however they tend to lack the distinct creamy coloured white-lipped marking (upper lip)(supralabials) of most A. hawkei and white labial markings common in most A. antarcticus. For example refer to the reddish coloured specimen from Dutchess, Qld., depicted on page 34 of this journal and in Hoser, (1995), pages 10-11 top, and compare with the specimens of A. hawkei depicted in Hoser (1995) pages 10-11 centre, the (different) specimens on the cover of Monitor 8 (3) 1997 (Hoser 1997a) and in Hoser (1989) or ones in this journal.
Acanthophis from near Camooweal, Queensland, tend to have heavy white markings on the lower supralabials, (upper lips) but unlike in A. hawkei from Anthony's Lagoon, NT, they do not quite form a distinct "white-lipped" appearance. Some reptile keepers have classified these snakes as "Barkly Adders" (=A. hawkei) and it is probably with these snakes that the Camooweal Acanthophis have closest affinity, noting that Camooweal is situated roughly on the edge of the black soil part of the Barkly tableland. This author regards Camooweal Acanthophis as A. hawkei.
Biology: Biological and captive breeding information about A. antarcticus is provided by the following author's: Carpenter et. al. (1978), Gilbertson-Middlebrook (1981), Hay (1972), Hoser (1981, 1982, 1983, 1984a, 1984b, 1985a, 1985b, 1985c, 1987, 1989, 1992, 1995, 1997a), Hoser and Williams (1991), Hudson (1979), Johnston (1987), Mirtschin (1976, 1982, 1985), Mirtschin and Davis (1991, 1992), Shine (1980, 1991), Swan (1990), Worrell (1972). Excellent photos of this species can be found in Ehmann (1992), Hoser (1989) (more than in any other publication to date), Hoser (1995), Wilson and Knowles (1988) and many other publications.
Mating behavior in A. antarcticus has been documented by Hoser
(1983, 1997a) and the behavior tends to be stereotyped in  line
with the accounts published by Carpenter and Fergusson (1977) Mating has
also been observed by this author in A. lancasteri, A. praelongus
and A. pyrrhus, with all species tending to behave in a similar
manner. Notable is a common trend that young inexperienced snakes tend
(on average) to take longer to effect a successful copulation than more
mature and experienced ones.
line
with the accounts published by Carpenter and Fergusson (1977) Mating has
also been observed by this author in A. lancasteri, A. praelongus
and A. pyrrhus, with all species tending to behave in a similar
manner. Notable is a common trend that young inexperienced snakes tend
(on average) to take longer to effect a successful copulation than more
mature and experienced ones.
Trade: Hoser (1991, 1993, 1996) discusses the legal and illegal trade of Australian reptiles, including Acanthophis, as well as conservation of these snakes. Persons within Australia contemplating trapping, studying or keeping these snakes, or any other Acanthophis and complying with the relevant state laws are referred to Hoser (1993, 1996).
Acanthophis antarcticus schistos Wells and Wellington, 1985
Wells and Wellington gave the name to a specimen from near Perth WA. They gave no other significant information or reason for their naming the snake "Acanthophis schistos". However most herpetologists recognise the western populations of A. antarcticus as having at least minor differences to those from the east. Therefore the name remains valid, at least as a subspecies. Until other information becomes available, the Western Australia/South Australian population of A. antarcticus which remains more or less continuous should be assigned to this subspecies. The remainder should be assigned to the nominate subspecies.
Storr, Smith and Johnstone (1986) and Bush et. al. present photos of live A. antarcticus schistos. Davis, et. al (undated) (about 1980), present photos of South Australian A. antarcticus schistos.
Captivity: Little has been recorded to date on this subspecies from Western Australia. Mirtschin and others have published extensively on South Australian A. antarcticus schistos.
Acanthophis barnetti sp. nov.
Holotype: A young adult male from Pukago (sometimes called "Pakago"), East Sepik Province, PNG Lat: 03° 52' Long: 142° 57', collected by W. H. Ewerson on 31st October 1964. Held at the Australian Museum, Sydney, R129223. Scalation: 120 ventrals, 37 single subcaudals, 11 paired, (48 total), the scale above the eye is not raised, or if so, only slightly. The general colouration is greyish brown (3 basic colours on the body being yellowish-brown, greyish-brown and blackish brown, arranged in somewhat indistinct bands). For colour details of the type specimen see the photo in this journal. There are no distinct markings on the upper face (supralabials) and it seems to be mottled all over, which is relatively unusual for PNG Acanthophis, (or at least certainly in the series held at the Australian museum). The front ventrals are dark grey with white at the posterior side, seeming almost like bands (one per scale).
Diagnosis: A medium sized Acanthophis from the northern part of New Guinea. The animal is readily distinguished from A. laevis by the fact that the supraocular scale (scale above the eye) is not raised in the same manner as in A. laevis and it's higher average ventral scale count (over 118 in A. barnetti). It is also separated from A. laevis by there being heavier dark pigmentation under the chin and near ventrals (evident in the type specimen of A. barnetti)(A. laevis has relatively little in this region of the body). A. barnetti is separated from A. rugosus by distribution (north of the central highlands, versus south) and the lack of rugosity on the head and neck. (Also see for A. laevis, A. rugosus and A. crotalusei, below).
It has been suggested that further studies of Acanthophis north of the New Guinea central highlands may ultimately result in all forms being treated as subspecies of a single "lowland" species (not laevis or rugosus). If such ultimately occurs, then the name barnetti should be treated as the nominate subspecies in favour of crotalusei.
Biology: Nothing known. No captive records known for the species.
Etymology: Named after Brian Barnett. Having devoted nearly 50 years to the study of reptiles, Barnett has kept and successfully bred many species of reptile in Australia, including being the first to do so for many species. In 1977, he formed the Victorian Herpetological Society which through his 21 year presidency of the society (ongoing in 1998) has helped many hundreds of people to keep and study reptiles.
Many people who first approached Barnett to obtain their first pet snake have long since completed tertiary studies and are now doing research of their own, as are many more who through Barnett's guidance and encouragement are similarly making new discoveries about Australasian herpetofauna. Barnett's achievements have also included the bringing in of a workable reptile licensing system in the State of Victoria, which while being far from perfect, has been vastly superior to the regime enjoyed in New South Wales over most of the past two decades, where his influence was not felt by the authorities. Barnett's wife of many years, Lani and children have also played an essential role in his herpetological efforts and the species A. barnetti is also named in their honour.
Acanthophis crotalusei sp. nov.
Holotype: An adult female at the Australian Museum, Sydney, Australia, R120878 (also with a tag no. 33682) from Madang, Madang District, PNG, Lat: 5° 12' Long: 145° 47'. Collected by H. G. Cogger on 19 May 1986. Scalation: 123 ventrals, 21 mid-body rows, 37 single subcaudals, followed by 5 paired, giving a total of 42 subcaudals, 6 supralabials, 7 infralabials, 3 suboculars. The scale above the eye is very raised. The head and neck scalation is slightly rugose.
For colouration details see photo in this journal. Colouration is a greyish-brown colour scheme with bands which appear to be indistinct (the snake was sloughing when killed and was not tampered with when inspected by this author, noting that markings would tend to be somewhat brighter after completing the slough). All supralabials and infralabials have distinct dark blotches in their centres. None of these blotches reach to the lip. the second last supralabial is very large. A distinct temporal line runs through the eye, apparently coordinating to an extent with the eye colour. The head is marginally darker than the neck. The belly has a mottled appearance but appears to be very dark. The tail has a yellow tip.
Paratypes: R15750, from Madang, Madang District, PNG, Lat: 5° 12' Long: 145° 47' collected by N. B. Blood. R120879, from Madang, Madang District, PNG, Lat: 5° 12' Long: 145° 47', Collected by H. G. Cogger on 20 May 1986. R121443, R122103 and R122104 all from Kar Kar Island, Madang District, PNG, Lat: 4° 37' Long: 145° 54' collected by G. Mengden and F. Parker in May 1986. All held at the Australian Museum in Sydney.
Details of R15750: young adult female, 45.5 cm snout-vent, 8 cm tail, 53.5 cm total length. 126 ventrals, 45 subcaudals (total), first 13 subcaudals single.
Diagnosis: A moderate sized Acanthophis believed to be widespread in New Guinea, although the current confirmed distribution is confined to the Madang area. (Further specimens in the collection at the Australian Museum conformed to this species, but time constraints prevented further inspection, including scale counts of these to confirm that they were in fact A. crotalusei).
This species is in many respects intermediate between A. laevis and A. barnetti. It is essentially similar in appearance to A. laevis from which it may usually be separated by the following characteristics, more black pigment on the upper labials, a more thick-set build in adulthood and higher average ventral count (under 118 in A. laevis, over 118 in A. crotalusei, refer to scale counts quoted by McDowall (1984)). Some but not all specimens of this species (A. crotalusei) have a well defined temporal line, particularly in front of the eye (as in A. barnetti). Separated from A. barnetti by the fact that A. crotalusei does not have distinct black lines running up the infralabials to the mouth (like in A. barnetti). A crotalusei tends to have a more raised supraocular scale than is usually seen in A. barnetti. Both species are also presently separated by distribution. Separated from A. rugosus by distribution and the substantially more rugose head and neck of A. rugosus (in adulthood at least - this author has not inspected any young A. rugosus). Illustration of this species (A. crotalusei) in life is shown in O'Shea (1996), p. 157 (lower).
Biology: Not known. Assumed to be similar for other Acanthophis. Likewise for captivity, although to date nothing is recorded.
 Etymology: Named after the author's dog, (itself named after a North
American genus of snake) who guarded the author's house and files against
break ins and raids for over 9 years.
Etymology: Named after the author's dog, (itself named after a North
American genus of snake) who guarded the author's house and files against
break ins and raids for over 9 years.
Acanthophis cummingi sp. nov.
Holotype: An adult female specimen in the Australian Museum, Sydney, Australia, R12438 from Yirrkala Mission, Darwin, NT. Lat: 12° 15' Long: 136° 53'. Scalation: 23 mid-body rows, 31 single subcaudals (at least)(this is not the complete number of subcaudals), 124 ventrals, about 56 bands on the body (excluding the head and tail). Black or dark coloured tip of tail. The type specimen was collected by W. S. Chaseling. For the colour of the type specimen, see the photo with this paper. Note that the specimen appears to have faded over time.
Paratype: Another adult female from the same locality, (Lat: 12°
15' Long: 136° 53'), R12552 held at the Australian Museum in Sydney.
23 mid-body rows, for the subcaudals, the 1st is paired, the next 17 are
single, 1 is paired, one is single then the rest  are
paired (running towards the tail tip) (this is not the complete number
of subcaudals), 121 ventrals. Black or dark coloured tip of tail. The paratype
was collected by W. S. Chaseling. Note: both the type and paratype presented
difficulties in counting scales accurately; hence no complete count of
subcaudals for either.
are
paired (running towards the tail tip) (this is not the complete number
of subcaudals), 121 ventrals. Black or dark coloured tip of tail. The paratype
was collected by W. S. Chaseling. Note: both the type and paratype presented
difficulties in counting scales accurately; hence no complete count of
subcaudals for either.
Diagnosis: For the colouration in life, see this magazine depicting a specimen from just south of Darwin, NT. It should be noted that in line with other Acanthophis, A. cummingi is extremely variable in colour, even within a single locality.
For many years A. cummingi have been mis-identified as A. antarcticus. (e.g. Gow 1977). In the last decade or so, many private reptile keepers have called these snakes "floodplain praelongus" in order to differentiate them from the "hill form" now known as A. lancasteri, which occurs in hilly areas of the Kimberley ranges and elsewhere. There is uncertainty as to the exact distributional status between both very similar forms. In spite of similarities between what is herein regarded as A. cummingi and A. lancasteri, it is proposed that two taxa are involved, hence the assigning of A. cummingi to the above described variant of Acanthophis. Genetic testing may help resolve the accurate status of the relationship between these snakes.
A. cummingi is a relatively large form of Acanthophis
and can usually (in life) be separated from A. lancasteri (and other
Acanthophis) by the following suite of characteristics. Scalation
ranges from smooth to very slightly keeled (less than is usually  the
case seen in A. lancasteri). Rugosity appears to be more prominent
in males than for females, a trend shared with A. lancasteri. Adult
A. cummingi in life almost always have distinct yellow bands, which
appear brighter and more pronounced when the snake flattens itself out,
sometimes giving it a flecked appearance. Specimens tend to have larger
and more distinct white markings on the lower supralabials (upper lip)
than is usually the case for A. lancasteri; refer to the paratype
as an example and see photos in this journal. They also appear to be marginally
more thick-set than A. lancasteri and are known to attain larger
sizes (length and weight - see below).
the
case seen in A. lancasteri). Rugosity appears to be more prominent
in males than for females, a trend shared with A. lancasteri. Adult
A. cummingi in life almost always have distinct yellow bands, which
appear brighter and more pronounced when the snake flattens itself out,
sometimes giving it a flecked appearance. Specimens tend to have larger
and more distinct white markings on the lower supralabials (upper lip)
than is usually the case for A. lancasteri; refer to the paratype
as an example and see photos in this journal. They also appear to be marginally
more thick-set than A. lancasteri and are known to attain larger
sizes (length and weight - see below).
Three live captive A. cummingi in Victoria had ventral counts of 121, 122 and 120, implying 120-124 is the approximate range for the species.
Some specimens of A. cummingi become greyish towards the head and upper neck regions, which is a trait shared with some A. lancasteri. A. cummingi is separated from all other Australian Acanthophis by known distribution. Contrary to earlier publications (e.g. Gow 1977), it is now accepted that there are no A. antarcticus at the top-end of the Northern Territory.
Maximum size known: This author has seen and photographed a specimen in captivity held by Chris Hay of Gisborne, Victoria, measuring 92 cm in total length (in 1997) and of substantial girth, weighing 800 grams (measurements confirmed by this author). As of April 1998, Hay reported that the snake had grown slightly since the earlier (1997) measurement had been taken. A specimen nearly as large was held by Stuart Bigmore at Lara, Victoria. This makes A. cummingi substantially larger than any A. lancasteri known (refer to Storr 1981, who gives a maximum snout-vent length for WA A. lancasteri in his study as 48.2 cm or Cogger 1992 who gives an average size of 40 cm and maximum of 70 cm (for A. praelongus, including A. lancasteri). This author has never seen an A. lancasteri in excess of 75 cm total length.
Venom Toxicity: No published research results are known. On 16/4/96, Chris Hay, an adult male reptile keeper in Gisborne, Victoria was bitten by a large (92 cm long) captive adult female A. cummingi (referred to above) from near Humpty Doo, NT, and was admitted to the Royal Melbourne Hospital in Parkville, Victoria. He was given 18,000 units of Death Adder anti-venom to neutralise the venom. This is three times that usually required to neutralise a Death Adder bite.
Known Distribution: A. cummingi, is believed to be restricted to the floodplain and adjacent regions of the far north of the Northern Territory in the vicinity of Darwin, including Fogg Dam and near the Marakai Floodplains where it is apparently very common. These are the only areas from where the species is currently known.
Captivity: No breedings of A. cummingi are known to this author. Relatively few are held in captivity. Those held by keepers Fred Rossignoli, Ringwood, Victoria, Stuart Bigmore, Lara, Victoria and Chris Hay, Gisborne, Victoria have presented no husbandry problems and are kept in the same manner as A. lancasteri and A. antarcticus by other keepers who have had success with those species. Lack of breeding in captivity to date (or records thereof), probably result from a lack of specimens in captivity as opposed to any inherent difficulties in breeding.
A case of cannibalism is known for the species. A large captive female of about 75 cm (total length), ate a male of about 60 cm (total length). There was no food in the cage at the time. The female digested the male in the same manner as usual food eaten
Etymology: Named after Fia Cumming, political reporter in 1998 with the Sydney Sun-Herald newspaper. In August 1981, she became the first journalist to report on corruption involving stolen reptiles within the NSW National Parks and Wildlife Service (NPWS). She followed up with newspaper stories over the following 15 years and her investigations into a kangaroo meat substitution racket involving senior NPWS officials and other reptile-related matters, culminated in material included in the book Smuggled-2 (Hoser 1996). That material became subject to a series of failed defamation actions against this author in 1996, which then led to a series of events culminating in the effective disbandment of most of the (now discredited) law enforcement arm of NPWS and introduction of a rational reptile licencing system in NSW for the first time ever in late 1997.
Without the investigations and reportings by Cumming, it is probable that no such overhaul of reptile and other wildlife laws in NSW would have ever occurred which would have continued to severely restrict ongoing herpetology and conservation in that state and by extension, throughout Australia. In effect, Cumming has possibly contributed more to the field of herpetology in Australia than any other non-herpetologist.
Acanthophis hawkei Wells and Wellington 1985
Known colloquially as the Barkly Adder, a sub-adult female was depicted on the front cover of Monitor 8 (3) 1997 (refer to Hoser (1997a)). Apparently it lives on black-soil plains and is probably the largest Acanthophis. In spite of the preceding statement, quotes of adult sizes in the literature are not matched by the sizes of specimens in museum collections or for that matter private facilities.
A. hawkei is closely related to A. antarcticus, with
which it was confused for many years. Distinguished from most other A.
antarcticus by the fact that the lower part of the supralabials (upper
lip scales) usually (but not always) tends to be creamish in  colour
without darker markings reaching the lip, giving it a jagged "white-lipped"
appearance (e.g. see photos in Barnett and Gow 1992 or Hoser 1989, 1995,
1997a).
colour
without darker markings reaching the lip, giving it a jagged "white-lipped"
appearance (e.g. see photos in Barnett and Gow 1992 or Hoser 1989, 1995,
1997a).
This jagged "white-lipped" appearance is relatively unusual in A. antarcticus and has never been seen to the same degree as is typical for A. hawkei. Excluding A. antarcticus, adult A. hawkei could not be confused with any other Australian Acanthophis.
Captivity: Breeding data for captive specimens is provided by Barnett and Gow (1992). Barnett quoted snout-vent lengths of offspring in a litter ranging from 193-207 mm, which is far in excess that recorded for any other species of Acanthophis, including A. antarcticus, lending weight to claims that this is the largest form of Acanthophis. The species has been bred in captivity in recent years by Brian Barnett in Victoria (again) and Roland Burrell in South Australia.
Acanthophis laevis Macleay, 1877
This species herein resurrected from the (relatively recent) synonymy of A. praelongus. Type locality Katow, PNG, Lat: 09° 06' Long: 143° 00'. (Katow is the old name for Mawatta on the Binaturi River in southern Trans-Fly of Western Province, PNG)(O'Shea, 1998). A. laevis is also separated from A. praelongus and A. lancasteri by it's average lower ventral count, (usually under 118 in A. laevis, and higher than that in A. praelongus).
The type specimen of A. laevis has not been inspected by this author, however an inspection of Acanthophis from the same locality and nearby areas conform with Macleay's description and this author has assigned all those snakes to A. laevis. The snake in question is substantially different to the A. praelongus described by Ramsay, which is presumably based on a north Queensland Acanthophis, from near Somerset, (Cape York) Queensland. Acanthophis laevis has smooth scales, while A. praelongus tends to have slightly keeled scales. Head patterning of both species is also usually radically different. For example compare the photo of A. praelongus from North Queensland (plate 380, Hoser, 1989) with the A. laevis shown here.
For most A. laevis seen by this author, the last supralabial and adjoining temporal shield have a distinct black blotch in the centre. Such markings have not been observed by myself in A. praelongus, where instead the darker markings in this region are not defined the same way and tend more to merge into that of the slightly lighter upper head.
A. laevis from the central and Western Highlands regions of the Island of New Guinea (including Irian Jaya) tend to have little in the way of darker head markings or blotches (above the mouth) except for those at the rear of the mouth (rear supralabials). This includes those from around "Katow", which appears to be a lowland locality. Those from more eastern highland areas, west to about Goroka often tend to have mottling on the forward supralabials, (refer also to photos on page 157 of O'Shea (1996)). While many species of Acanthophis have a raised scale above the eye (supraocular), particularly in younger specimens, none have this trait to the same extent as A. laevis, which retain the trait into adulthood, when the raised scale remains prominent.
A. laevis though usually associated with highland areas is known from lowland areas in Western Province and Irian Jaya (e.g. "Katow"). McDowall (1984) noted a lower average ventral count for this species, which has been confirmed by my own counts of specimens at the Australian Museum in Sydney (e.g. R14352 from Goroka, PNG, with 115 ventrals (adult female), versus the low 120's for most other Acanthophis including Queensland A. praelongus). Macleay's type specimen had 113 ventrals (Macleay 1877).
McDowall (1984) and O'Shea (1996) suggest that highland New Guinea Acanthophis, herein regarded as A. laevis have strong affinities to A. antarcticus from Australia. This author disagrees with that conclusion. Besides the obvious difference of the very raised scale above the eye (noted by both authors), head markings tend to be quite unlike most A. antarcticus from Australia. Furthermore, this author has yet to see any specimens from the New Guinea highlands that attain the size and weight of some Australian A. antarcticus. Confusion within Australia between A. antarcticus and A. praelongus may have led to the above authors making their statements, meaning to imply similarity to A. praelongus (from Queensland), instead of the more southerly distributed A. antarcticus.
Reference by O'Shea (1996) of a localized "small montane race (to 300 mm)" are probably of this species (A. laevis). All anecdotal evidence, suggests that A. laevis is the smallest of the four species of Acanthophis known from New Guinea (this paper), including that of O'Shea (1996) and McDowall (1984). McDowall (1984) noted that Acanthophis from south-western PNG (near Australia), have little in common with those from Queensland, Australia, further confirming the different specific nature of New Guinea Acanthophis. That McDowall was referring to A. laevis is not in doubt as he identifies it as "a form with reduced ventral count, reduced black pigmentation and the temporolabial entering the mouth". Inspection of specimen number R23960 at the Australian Museum confirmed McDowall's assertion that A. laevis also on occasion occurred away from the central highlands, including south-western PNG.
Data for R23960 is as follows:- Collected at Balimo, Aramia River, Western District, PNG, Lat: 08° 01' Long: 142° 57' on 3 November 1963. Identified by this author as A. laevis. Age: adult. Snout-vent 39.5 cm, Tail 10.5 cm, Total length 50 cm. Sex: male. Scalation is smooth with 111 ventrals (2 of which were paired), 34 single subcaudals, 14 paired, (48 total). Other specimens from the same locality are held at the Australian Museum.
Lindgren (1975), plate 88 depicts a head photo of a snake this author believes is probably A. laevis in life. However it's facial markings are not like the A. laevis at the Australian Museum. It is believed that the non-black dark pigment tends to fade faster than the black pigment in preserved animals; this trait is believed to be common to all Acanthophis. The point is noted here as a lack of black pigment in A. laevis, may make specimens fade more than other Acanthophis species.
Biology: Little known, but presumed to be similar to that of other Acanthophis. Worrell (1972) records this species as being most active at the end of the wet season which roughly parallels the activities of Acanthophis in northern Australia by the author's own investigations and what was said by O'Shea (1996) in his summary of New Guinea Acanthophis. Lindgren (1975) states Acanthophis in PNG range up to nearly 2000 metres. It is assumed that these high altitude Acanthophis are probably A. laevis, at least in the highland areas west of Goroka and into Irian Jaya.
Captivity: Nothing known by this author, although it is assumed that specimens are in captivity in the northern hemisphere.
Acanthophis lancasteri lancasteri Wells and Wellington 1985
Originally described by Wells and Wellington as "Acanthophis lancasteri", based on a specimen from near Halls Creek, WA. The same type of animal is depicted in Hoser (1989), plates 378, 379 listed there as A. praelongus from Kunnanurra, WA, (which is not far from Halls Creek) and in Storr, Smith and Johnstone (1986), p. 127. So far all Acanthophis from the East Kimberley region seen by this author can be readily assigned to this species. Wells and Wellington differentiated A. lancasteri from A. praelongus, including giving it's distribution as being the top end of the Northern Territory and Western Australia (namely the hilly tropical north, including the Kimberley Ranges and Arnhem land escarpment). However they failed to give any separating characteristics between the species.
Perhaps the most readily identifiable difference is in ventral colouration.
A. praelongus tends to have well defined brown spotting on the ventral
scales, which is relatively unusual in A. lancasteri. A. praelongus
usually has two well defined white  markings
which are more or less triangular in shape on the lower supralabial scales
(upper lip). A lancasteri (from WA at least) rarely has such markings,
or if present, they are usually not clear and well defined, but rather
mottled in appearance, tending to merge with the adjacent colour. (Both
species have well defined white markings on the infralabials).
markings
which are more or less triangular in shape on the lower supralabial scales
(upper lip). A lancasteri (from WA at least) rarely has such markings,
or if present, they are usually not clear and well defined, but rather
mottled in appearance, tending to merge with the adjacent colour. (Both
species have well defined white markings on the infralabials).
This author has also been told that A. lancasteri has a higher average ventral count than A. praelongus, but has not seen sufficient data to confirm this assertion. Storr (1981) gives a range of 122-134 (N=12) for A. lancasteri (which he calls praelongus). Ramsay (1987) gives a number of "about 120" for the original North Queensland A. praelongus, which is just outside the range quoted by Storr for his limited sample of A. lancasteri. In 1980, this author counted a Cairns, Queensland, A. praelongus as having 124 ventrals.
A lancasteri appears to be restricted to rocky and hilly habitats or adjacent areas and seems to be most common in areas where Triodia grasses dominate. My own experiences in the East Kimberley indicate the species is extremely common where such conditions occur, but rare or absent elsewhere. Personal communications from people who have collected "praelongus" type Acanthophis in the Northern Territory indicate a similar situation usually occurs there.
Longmore (1986) records no Acanthophis (of any species) in a distinct area of the Gulf of Carpentaria running several hundred kilometers south from the southern most part of the gulf, although Acanthophis are known from the islands of the gulf, including Groote (lancasteri) and Mornington (species not known). This apparent gap separates A. lancasteri from A. praelongus in north Queensland.
A trend noticeable in some specimens of A. lancasteri is darkening of the head and neck area. An example of this is seen in Hoser (1989), plate 378. This feature is not unique to this species, also being seen in Acanthophis wellsei the Pilbara region of Western Australia and possibly other forms as well (also see Wilson and Knowles (1988), p. 330. In A. lancasteri this trait is seen in populations throughout the range of the species, but becomes more pronounced in populations from the eastern section of the Northern Territory (see A. lancasteri bottomi below).
The nominate subspecies A. lancasteri lancasteri is herein confined to Western Australia and probably adjacent western sections of the Northern Territory (see below).
Captivity: A. lancasteri is very hardy in captivity and readily takes to feeding on mice. Male specimens kept by this author (in Sydney) from Kunnanurra and Turkey Creek, Western Australia presented no problems until stolen in 1984. At least one remained alive and healthy at Taronga Zoo, Sydney until at least 1992, when it was photographed by the author, meaning it had been held captive at that stage for 9 years. It had been adult when caught. Cannibalism has been recorded for this species, although it is regarded by this author as being relatively unusual.
Breeding: Has been bred in captivity a number of times, perhaps most notably by keeper Rob Valentic, of Greensborough, Victoria, whose snakes were identified as A. lancasteri or "hill praelongus". Valentic has detailed records of his results and is expected to publish details of them. A photo of one of Valentic's females giving birth in 1996 was published by Hoser (1997b) and is also published in this journal (see Valentic (1998)).
Acanthophis lancasteri bottomi subsp. nov.
Holotype: An adult female specimen in the Australian Museum in Sydney, Australia. R26274. Collected at Angurugu Mission, Groote Eylandt, Gulf of Carpentaria, Northern Territory, Australia, Lat: 13° 58' Long: 136° 27', by D. Levitt.
Paratype: An adult female from Groote Eylandt, Gulf of Carpentaria, Northern Territory, Australia. Lat: 13° 59' Long: 136° 28', collected by H. E. Warren. R10218 held at the Australian Museum in Sydney. Details of paratype: Snout-vent: 53.5 cm, Tail: 8.5 cm, Total length 62 cm, 129 ventrals, 24 single subcaudals, 18 paired/divided subcaudals, 42 total subcaudals.
Diagnosis: Essentially similar to the type subspecies A. lancasteri lancasteri. A moderate sized Acanthophis with strongly rugose scales on the head and neck. Young specimens have distinct bands which tend to fade with age, (based on observations of a series of specimens at the Australian Museum). For colouration details see the photo with this paper, but note the fact that the animal's colour has faded since death. Ventrally there are few markings and the belly is a lightish yellowy cream in colour. (The paratye has some darker flecks on the ventral surface). Distinguished from A. lancasteri lancasteri by it's strongly darkened head, which appears to be diagnostic of adults of this race. This subspecies (bottomi) also appears to occur on the adjacent Australian mainland, although in some areas, it forms apparent intergrades with the type subspecies and in some localities specimens assignable to either subspecies may occur.
Ecological notes: Little known. Most abundant in hilly areas. Assumed to have similar habits to the type subspecies.
Captivity: Nothing recorded.
Etymology: Named after investigative journalist Robert Bottom, author of several best-sellers about organised crime in Australia. In the mid 1980's he did a series of reports about corruption involving fauna officials in New South Wales. In 1991 he reported on Police corruption in Victoria a full twelve months before other "mainstream" journalists dared run with the story.
Acanthophis praelongus Ramsay, 1877
Herein restricted to the Cape York region of Queensland and adjacent areas. Refer to above descriptions of A. laevis and A. lancasteri. Populations of Acanthophis from Western Australia and the Northern Territory formerly referred to as this species are now classified as A. lancasteri. Populations of Acanthophis from New Guinea and other Islands north of Australia that may have been referred to as this species are no longer regarded as such (see for A. barnetti, A. crotalusei, A. laevis, above and A. rugosus below).
Captivity: A. praelongus has been bred in captivity by Roy Pails of Ballarat, Victoria, Andrew Lowry of Brighton, Victoria and others. Young are substantially smaller at birth (on average) than for A. lancasteri which have been bred by Rob Valentic of Greensborough. Excellent photos of live A. praelongus appear in Hoser (1989, 1995). Young appear to be more difficult to raise than for other Acanthophis, including A. lancasteri.
The Lowry breeding was of two Cardwell, Queensland, A. praelongus which resulted in six live young averaging just 12-13 cm in total length.
Acanthophis pyrrhus Boulenger, 1898
Type locality Station Point NT.
Photos of West Australian A. pyrrhus (armstrongi) appear in Hoser (1989, 1995) and Storr (1981). Northern Territory A. pyrrhus is depicted in Cogger (1992).
Captivity: Breeding data for captive specimens is provided by Fyfe and Munday (1988) and Gow (1981). Photos of mating A. pyrrhus are published in Glasby et. al. (1993), and Shine (1991). Cannibalism for this species has been recorded several times, indicating that it is probably more prone to this behavior than any other Australian Acanthophis.
Acanthophis pyrrhus armstrongi Wells and Wellington 1985
In 1985, Wells and Wellington assigned all Western Australian A.
pyrrhus to a new species, namely "A. armstrongi".
That they intended placing all A. pyrrhus from Western Australia
into the new species is confirmed by their statement 'Storr (1981:207-208)
provided a description of a species from north-western Australia that he
regarded as Acanthophis pyrrhus. 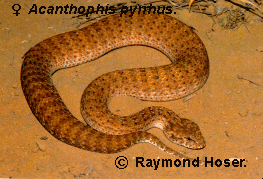 However,
we consider that this is really an undescribed species, herein named Acanthophis
armstrongi, and that the species Acanthophis pyrrhus is confined
to central Australia. Acanthophis armstrongi is believed confined
to the Pilbara and Kimberley regions of Western Australia and can be identified
by referring to the illustrations in Storr (1981: fig 3) and Gow (1983:
Plate 15, (upper), specimen from Port Hedland, Western Australia vide Gow,
Pers. comm.).'
However,
we consider that this is really an undescribed species, herein named Acanthophis
armstrongi, and that the species Acanthophis pyrrhus is confined
to central Australia. Acanthophis armstrongi is believed confined
to the Pilbara and Kimberley regions of Western Australia and can be identified
by referring to the illustrations in Storr (1981: fig 3) and Gow (1983:
Plate 15, (upper), specimen from Port Hedland, Western Australia vide Gow,
Pers. comm.).'
The type specimen of "Acanthophis armstrongi" was a snake collected 5 km east of Giralia, Western Australia, and is an adult in the Western Australian Museum, number R61357. The name "armstrongi" has since erroneously been used to describe a previously undescribed form from the hilly parts of the Pilbara region south of the Great Sandy Desert, including those from Millstream, Pannawonica and 60 km NNW of Newman, Western Australia (e.g. Hoser 1997).
These snakes, herein referred to as A. wellsei are radically different from the more northern A. pyrrhus, including those from Port Hedland, WA. As Wells and Wellington clearly indicated they were referring to a snake known as A. pyrrhus and that it was the form depicted in Storr (1981) and Gow (1983), which is still recognised by all other Australian herpetologists as the species A. pyrrhus (or close variant thereof) rather than the other form, the name armstrongi can clearly only be applied to A. pyrrhus, either as a junior synonym, subspecies, or as per Wells and Wellington a sibling species depending on one's taxonomic judgements.
Also note that the A. pyrrhus depicted in Storr 1981 is from Giralia, the type locality given by Wells and Wellington for "armstrongi". Thus armstrongi is not a valid name for the previously undescribed form and it's use to describe that animal is not correct. That form has been described later in this paper as A. wellsei.
Within the Pilbara region, A. wellsei appears restricted to the hilly parts of the Hamersley Ranges and immediately adjacent areas, while A. pyrrhus armstongi is found in flatter more arid areas to the north, south and east. The exact distribution of both types of Acanthophis in the Pilbara is not known. Museum collections appear to have substantial gaps in distributional samples.
This author regards A. pyrrhus from the Great Sandy Desert of WA and adjacent areas, including coastal parts of the Pilbara as being sufficiently different from the nominate form to be given subspecific status.
Specimens of A. pyrrhus armstrongi observed by this author in life appear to have more yellow colouring dorsally than those seen from central Australia, although whether this is a general trend difference between both forms is not yet known.
Acanthophis rugosus Loveridge, 1948
Type locality Merauke, Irian Jaya (south coast of New Guinea). The type specimen has not been inspected by this author. However an adult specimen in the Australian Museum R147655 with the same locality data is obviously the same species.
Upon viewing the Acanthophis at the Australian Museum spirit house, the animal R147655 was immediately retrieved as being like no other Acanthophis in the collection. That it's collection locality data matched that of specimen number 22812 at the Museum of Comparative Zoology (the type) came as no surprise.
Unlike other species from the island of New Guinea, A. rugosus has very strongly rugose scales on it's head and neck. The scale above the eye, does not appear to be raised as in A. laevis. Markings on the labials and head differ from those of other New Guinea Acanthophis, however other A. rugosus may display different head markings. At this stage A. rugosus is not known from any other locality. Cogger (1983) declared rugosus a junior synonym of praelongus. Neither species is remotely similar. It is assumed that Cogger had not inspected type specimens or others from the same localities. A. laevis from the Highland regions of the Irian Jaya side of the border seen by this author have looked similar to the one pictured with this paper.
Acanthophis wellsei sp. nov.
 Holotype:Held at the Western Australian Museum from Wittenoom Gorge,
WA, Lat: 22° 15' Long: 118° 23', R8886.
Holotype:Held at the Western Australian Museum from Wittenoom Gorge,
WA, Lat: 22° 15' Long: 118° 23', R8886.
Paratypes:R21538 also from Wittenoom Gorge, WA, Lat: 22° 14' Long: 118° 20'; R17121 and R18493 from Wittenoom, WA, Lat: 22° 20' Long: 118° 19'; R67921 from 31 km SE of Mount Meharry Lat: 23° 10' Long: 118° 54'; and R56097 from Marandoo, WA, Lat: 22° 38' Long: 118° 07'.
Diagnosis: Known from Millstream and adjacent parts of the Pilbara region of Western Australia, including Pannawonica and 60 km NNW of Newman, WA, where it is very common (Ball 1993). Distribution appears to be centred on the Hamersley Range area. It has been up until now an undescribed form. Many specimens tend to have black bands and darkening of the head (usually black), although this is not a diagnostic trait of the species as some specimens are not marked this way (see Ball 1993).
A. wellsei appears to be most closely related to A. lancasteri and/or A. pyrrhus, and tends to have smooth to moderately rugose scales, particularly on the sides. Acanthophis wellsei can in all cases known to this author, be distinguished from A. pyrrhus by having two prefrontals as opposed to four in A. pyrrhus (Bush 1988). The head of this species appears to be "deeper" than seen in A. pyrrhus and the side of the head does not flare below the eye as in A. pyrrhus. In these respects it is like A. lancasteri. A. wellsei is unlikely to be confused with any other Acanthophis.
Distribution of this species appears restricted to the range areas around the Hamersleys and Chichester Range of the Pilbara, although it does extend to lower areas nearby. Coastal areas to the north and east are evidently populated by A. pyrrhus. To date no areas of sympatry are known. Bush (1988) speculated that hybridisation between the two forms may occur. Further survey work in the Pilbara is required to fully resolve the distributional status of both forms.
Captivity: The species has been bred in captivity. Photos of the snake in life are shown in this journal and can also be seen in Ball (1993) as well as in Mirtschin and Davis (1992). The author understands numbers of this snake are being held captive at the present time, both in WA and the eastern states.
Cannibalism has not been recorded but based on the fact the species is similar to both A. pyrrhus and A. lancasteri, both of which are known to have cannibalistic tendencies, the habit is likely to be observed in A. wellsei. In terms of general husbandry matters, private keepers have not indicated problems.
An instance of mite infestation in a long-term captive reported to this author was cured without adverse incident on the captive snake.
Taxonomic History: In 1981, Glen Storr of the Western Australian Museum published the results of his study of Acanthophis in Western Australia (Storr 1981). The study was apparently based on preserved museum specimens. Based on his records published for A. pyrrhus in that paper, he clearly observed preserved specimens of A. wellsei but placed them within the earlier described species. This fact is deduced from his listing 11 out of 33 specimens of "A. pyrrhus" having two prefrontals, which appears to be diagnostic for A. wellsei as well as the locality information given for specimens examined as some locations included areas now known to only have A. wellsei. Type data given here was derived from that paper.
In 1991 the Western Australian Museum was supplied with two specimens of A. wellsei (Ball 1993). Other specimens were found by Dave Robertson and Brian Bush. Bird (1992) reported Ken Aplin of the Western Australian Museum as having discovered the snake. In 1993, the Second World Congress of Herpetology was told that Aplin would soon be publishing a description of the snake. After further effluxion of time it became clear that Aplin had chosen not to publish a description of the species as it was thought that Wells and Wellington had already published a description in 1985 and therefore the species was thought to already have a name ("armstrongi").
In 1997-8 when doing a taxonomic review of Acanthophis, this author obtained a copy of the Wells and Wellington paper and noted that they had in fact described "A. armstrongi" as a Pilbara death adder. However what had apparently been overlooked was that the snake described by Wells and Wellington had not been the undescribed form of Acanthophis, but rather the local variant of what is commonly known as A. pyrrhus.
Bush (1998) confirmed that the snake described by Wells and Wellington was not the undescribed form herein described as A. wellsei. This fact is further confirmed by referring directly to the Wells and Wellington paper and the fact that the snake was apparantly unrecognised by all until the early 1990's. The Death Adders from Giralia, WA are not the formerly undescribed form, but rather A. pyrrhus, or what has been recognised as such (noting Giralia as the type locality for "A. armstrongi")(also see photo in (Storr 1981) of an A. pyrrhus from Giralia, WA).
This author has been in regular contact with the Western Australian Museum staff for many years and received correspondences from them implying that they may undertake and publish a second review of the genus Acanthophis (e.g. Smith 1997), the first review being that of Storr (1981). It is noted that a time frame of over 6 years has elapsed since the undescribed Pilbara Acanthophis was originally found by scientists and staff at the Western Australian museum. It is noted that to date they have chosen not to describe it as a new species.
Further noting the conflicting views from Western Australian herpetologists over their impressions of the true taxonomic status of the previously undescribed Pilbara Acanthophis, (e.g. Storr 1981, Bird 1992), this author has decided to publish a formal description of the snake as a new species here. It is not proper for one group of workers to apparantly monopolise a species and then fail to publish on it within an acceptable period, noting that 6 years is deemed by this author to be an unacceptable delay. Of course other authors including those from Western Australia may dispute the assertion published here that A. wellsei sp. nov. is in fact a separate and distinct form of Acanthophis. Nothing in the above should be taken as a personal criticism of anyone at the WA Museum, all of whom this author holds in highest regard.
Etymology: Named after Richard Wells. He is a highly knowledgeable and talented herpetologist who in the mid 1980's published a series of controversial taxonomic works, described by some critics as "reckless" (cited at the end of this paper).
An attempt was made by a number of high profile herpetologists to have the International Commission of Zoological Nomenclature (ICZN) to have the relevant works of Wells and co-author, C. Ross Wellington formally suppressed. Such did not occur (Shea 1998) and many of the names proposed by the pair have found their way into widespread acceptance (e.g. Antaresia, Morelia spilota mcdowelli). Other taxonomic judgements by the two have either been disagreed with or following further research found to be in error. However such a situation is not unusual in taxonomy, noting for example similar judgements being made against the taxonomic works and conclusions of Storr (e.g. Bohme 1992), Sprackland (e.g. Shea 1998) and others, whom are still highly regarded and respected within their areas of publication. Therefore disagreement by peers with the conclusions of Wells and Wellington should not be in itself relied upon to cast adverse judgement upon the pair.
Disagreements about taxonomic conclusions are part and parcel of the science of zoology. In the main the papers of Wells and Wellington assigned species names to well recognised taxa that until then did not have such names and as such their taxonomic judgements are not in doubt.
In recent years there seems to have been an attempt by some in the "herpetological establishment" to wipe any references to Wells and Wellington from the record, perhaps encapsulated in the attempt by Sprackland et. al. to wipe the name Varanus keithhornei (Wells and Wellington 1985) in favour of his later proposed name Varanus teriae (Sprackland 1991), which violates the basic ICZN rule of "priority". That case being before the ICZN in 1998. Refer to Shea (1998), or other relevant articles within the Bulletin of Zoological Nomenclature published in 1997-8 (cases 3042-3043). Another example is the apparent suppression of a name given by Wells and Wellington in 1985 to the western form of "Children's Python", subsequently re-named as "stimsoni" again in violation of the ICZN priority rule.
The name wellsei has been chosen to help ensure that recognition of the substantial contribution to herpetology in Australia of Richard Wells remains in the future and is not "erased" from the historical record. This should not be taken as a carte-blanche endorsement of Wells' taxonomic judgements in all matters.
Acanthophis woolfi sp. nov. To view a photo of a specimen of this species - click here.
Holotype: Held at the Queensland Museum from "Mount Isa area", Queensland, Lat: 20° 44' Long: 139° 29'; R61449.
Paratype: Held at the Queensland Museum from "Mount Isa area", Queensland, Lat: 20° 44' Long: 139° 29'; R61538
Diagnosis: Similar in most respects to both A. hawkei and
A. antarcticus, to which this form is obviously most closely related.
 A.
woolfi may usually be distingushed from the previous two species by
the relative lack of white on the supralabials, (see photos of live specimens
on page 34 of this journal).
A.
woolfi may usually be distingushed from the previous two species by
the relative lack of white on the supralabials, (see photos of live specimens
on page 34 of this journal).
Scalation can be moderately rugose and colouration varies, although reddish/orangey colour forms are most common, which no doubt reflects the dominant soil colour in their range. In line with A. hawkei, young A. woolfi tend to have slightly darker and more intense colours than adults, indicating colour change through life.
Known Distribution: The area bounded by Mount Isa, Cloncurry, Dutchess
and Dajarra, all in north-west Queensland. To the north-west, A. hawkei
appears to take over, while to the west A. pyrrhus becomes the species
encountered. Where A. woolfi occur, no other Acanthophis
are known. There is a possibility that A. woolfi may occur
further south and east of the range indicated here. Museums throughout
Australia appear to lack in Acanthophis specimens from north-west
Queensland. This most probably reflects a lack of collecting rather than
any actual rarity. A. woolfi appears to be reasonably common in
the area between Dutchess (Lat: 21° 21' Long: 139° 52') and Dajarra
(Lat: 21° 42' Long: 139° 31'), with herpetologists reporting seeing
up to five in a single night's driving.
Captivity: Of three specimens known by this author to have been kept in captivity, none presented any husbandry problems and each lived for some years. Qld/NPWS refused to give this author a permit to collect and keep this species, but did grant a permit to collect, hold, photograph, then release any form of reptile in that state. It is hoped that someone is eventually allowed to hold these animals legally so husbdandry and ecological questions can be answered.
Etymology: Named after herpetologist Paul Woolf. He has assisted many other reptile people for some years through his involvement with the Herpetological Society of Queensland and other groups. For his efforts he's been unlawfully harassed by wildlife officials in Queensland and New South Wales, the latter of which is where he resided in 1998.
Various assistance's, mainly in the form of providing literature, locality data or providing of study specimens in their care (including museum specimens), by Brian Barnett, Stuart Bigmore, Frank Bonaccorso, Brian Bush, John Cann, Allen Greer, Chris Hay, Bill Love, Andrew Lowry, Samuel McDowall, Mark O'Shea, Ross Sadlier, Grant Turner, Robert Valentic and Paul Woolf. Many others who freely allowed the author to observe live snakes in their care and provided other assistance's have been omitted from this list.
LITERATURE CITED
Ball, S. 1993. Further data on the Pilbara Death Adder - A suspected New Species, Monitor, Bulletin of the Victorian Herpetological Society, 5 (1):5-10.
Barnett, B. F. and Gow, G. F. 1992. The Barkly Tableland Death Adder, Acanthophis antarcticus, Monitor, Bulletin of the Victorian Herpetological Society, 4 (1):13-23.
Bird, P. 1992. Add an adder, Australiasian Post, 18 January.
Bohme, W. 1991. The identity of Varanus gouldii (Gray, 1838), and the Nomenclature of the V. gouldii-Species Complex. Mertensiella 2:38-41.
Boulenger, G. A. 1898. Description of a new death adder (Acanthophis) from central Australia. Annals Magazine of natural History, 7 (2):75.
Bush, B. 1998 E-mail to Raymond Hoser, April 8, 1 p..
Carpenter, C. C. and Ferguson, G. W. 1977. Stereotyped sexual behavior in reptiles, in Biology of the Reptilia, Academic Press, Vol. 7:335-554.
Carpenter, C. C., Murphy, J. B. and Carpenter, G. C. 1978. Caudal luring in the Death Adder (Acanthophis antarcticus (Reptilia, serpentes, elapidae)), Journal of Herpetology, 12:574-577.
Cogger, H. G. 1983. Zoological Catalogue of Australia (1) Amphibia and Reptilia, Australian Government Publishing Service, Canberra, ACT, Australia. 319 pp.
Cogger, H. G. 1992. Reptiles and Amphibians of Australia, Revised Edition, Reed Publishing, Sydney, NSW, Australia. 775 pp.
Coventry, A. J. 1997. Letter to Andrew Lowry, 2 May, 2 pp..
Coventry, A. J. and Robertson, P. 1991. The Snakes of Victoria, Department of Conservation and Environment, East Melbourne, Victoria, Australia. 75 pp.
Davis, R., Fennell, P. and Mirtschin, P. (undated)(about 1980), Poisonous Snakes of South Australia's Eyre Region, Whyalla News, South Australia. 27 pp.
Ehmann, H. 1992. Encyclopedia of Australian Animals: Reptiles, Angus and Robertson, Pymble, NSW, Australia. 510 pp.
Fairley, N. H. 1929. 'The present position of snake-bite and the snake bitten in Australia.', The Medical Journal of Australia. March 9th:296-313.
Fyfe, G. and Munday, B. 1988. Captive breeding of the Desert Death Adder (Acanthophis pyrrhus), Herpetofauna, 18 (2):21.
Gilbertson-Middlebrook, K. 1981. 'A note on Death Adder mortality following the laying of poison', Herpetofauna, 12 (2):36.
Glasby, C. J., Ross, G. J. B., and Beesley, P. L. (eds) 1993. Fauna of Australia. Vol. 2A Amphibia and Reptilia. Australian Government Publishing Service: Canberra, ACT, Australia. 447 pp.
Gow, G. F. 1977. Snakes of the Darwin Area, Museum and Art Galleries board of the Northern Territory, NT, Australia. 29 pp.
Gow, G. F. 1981. Notes on the Desert Death Adder (Acanthophis pyrrhus) Boulenger 1898, with the first reproductive record. Northern Territory Naturalist, 4:21-22.
Hay, M. 1972. Notes on the growth and breeding of Acanthophis antarcticus', The Australian Herpetological Society Journal, 4 (4):14-15.
Hoser, R. T. 1984a. Search for the Death Adder, Notes From NOAH, 11 (9):12-14.
Hoser, R. T. 1989. Australian Reptiles and Frogs, Pierson and Co., Sydney, NSW, Australia. 238 pp.
Hoser, R. T. 1991. Endangered Animals of Australia, Pierson and co., Sydney, NSW, Australia. 240 pp.
Hoser, R. T. 1997a The mating behavior and breeding of Australian Death Adders, Genus: Acanthophis (Serpentes: Elapidae), Monitor - Journal of the Victorian Herpetological Society 8 (3):135-144 and cover.
Hoser, R. T. 1997b Herpetology in Australia: Some good news, The Reptilian 5 (4):19-23. *
Hoser, R. T. and Williams, C. 1991. Snakes swallowing their own teeth, Herpetofauna, 21 (2):33. *
Hudson, P. 1979. On the breeding and birth of Death Adders in captivity, Herpetofauna, 11 (1):11-13.
Johnston, G. R. 1987. Reproduction and growth in captive Death Adders Acanthophis antarcticus (Squamata: Elapidae). Transactions of the Royal Society of South Australia, 11 (1-2):123-125.
Kim, H. S. and Tamiya, N. 1981. Isolation, properties and amino acid sequence of a long chain neurotoxin, Acanthophis antarcticus B, from the venom of and Australian snake (the common death adder Acanthophis antarcticus), Biochemical Journal, 193:899-906.
Lindgren, E. 1975. Wildlife in Papua New Guinea, Frederick Muller, London, UK. 196 plates.
Longmore, R. 1986 Atlas of Elapid Snakes of Australia, Australian Government Publishing service, Canberra, ACT, Australia. 120 pp.
Loveridge, A. 1948. New Guinean reptiles and amphibians in the Museum of Comparative Zoology and United States National Museum, Bulletin of the Museum of Comparative Zoology, 101 (2):305-430.
Macleay, W. 1877. The ophidians of the Chevert Expedition. Proceedings of the Linnaen society of New South Wales, 2:33-41.
McDowall, S. B. 1984. Results of the Archbold Expeditions. No. 112. The Snakes of the Huon Peninsula, Papua New Guinea, American Museum Novitates, 2775:1-28.
Menkhhorst, P. 1994. Letter to Geoffrey Allen, DCNR Mildura, February 21, 1 p..
Mirtschin, P. J. 1976. Notes on breeding Death Adders in Captivity, Herpetofauna, 13 (2):14-17.
Mirtschin, P. J. 1982. Further notes on breeding Death Adders (Acanthophis antarcticus) in captivity. Herpetofauna, 13 (2):14-17.
Mirtschin, P. J. 1985, An overview of captive breeding of Common Death Adders Acanthophis antarcticus (Shaw) and it's role in conservation, in Grigg, G., Shine, R. and Ehmann, H. (eds), Biology of Australasian Frogs and Reptiles, Royal Zoological Society of New South Wales:505-509.
Mirtschin, P. and Davis, R. 1991, Revised Edition, Dangerous Snakes of Australia, Ure Smith Press, Sydney, NSW, Australia. 208 pp.
Mirtschin, P. and Davis, R. 1992, Snakes of Australia - Dangerous and Harmless, Hill of Content, Melbourne, Australia. 216 pp.
O'Shea, M. 1996. A Guide to the Snakes of Papua New Guinea. Independent Group Pty. Ltd., Port Moresby, PNG. 251 pp.
O'Shea, M. 1998 E-mail to Raymond Hoser, April 13, 1 p.
Ramsay, E. P. 1877. Description of a supposed new species of Acanthophis from North Australia, Proceedings of the Linnean Society of New South Wales, 2:72-74.
Shaw, G. and Nodder, F. P. 1802. Boa antarctica. Naturalist's Miscellany:13, pl. 535.
Shea, G. 1998. Comments on the proposed conservation of the specific name of Varanus teriae Sprackland, 1991 (Reptilia, Squamata) (Case 3043; see BZN 54: 100-103, 250-251), Bulletin of Zoological Nomenclature 55 (1):38-39.
Sheumack, D. D., Howden, M. E. H. and Spence, I. 1979. Isolation and partial characterisation of a lethal neurotoxin from the venom of the Australian Death Adder (Acanthophis antarcticus), Toxicon 17:609.
Shine, R. 1980, Ecology of the Australian Death Adder Acanthophis antarcticus (Elapidae): Evidence for convergence with the viperidae. Herpetologica, 36 (4):281-289.
Shine, R. 1991, Australian Snakes - A Natural History, Reed Books, Sydney, NSW, Australia. 223 pp.
Smith, L. A. 1997. Fax to Raymond Hoser, January 16, 1 p.
Stettler, P. H. 1985. Australian Death Adders - Remarks concerning the post-embryonic development of Acanthophis antarcticus (Shaw, 1794), Litteratura Serpentium, English Edition, 5 (5):170-190.
Storr, G. M. 1981. The genus Acanthophis (Serpentes: Elapidae) in Western Australia, Records of the Western Australian Museum, 9 (2):203-210.
Storr, G. M., Smith, L. A. and Johnstone, R. E. 1986. Snakes of Western Australia. Western Australian Museum, Perth, WA, Australia. 197 pp.
Swan, G. 1990. A field guide to the Snakes and Lizards of New South Wales, Three Sisters Productions, Pty, Ltd, Winmalee, NSW, Australia, 224 pp.
van Woerkom, A. B. 1985. Postscript to the article "on the question of immunity of snakes", Litteratura Serpentium, English Edition, 5 (6):233.
Valentic, R. 1998. Notes on rearing Australian Death Adders Genus Acanthophis, Monitor - Journal of The Victorian Herpetological Society, 9 (2):32, 42-47.
Wells, R. W. and Wellington, C. R. 1983. A synopsis of the class Reptilia in Australia, Australian Journal of Herpetology, 1 (3-4):73-129.
Wells, R. W. and Wellington, C. R. 1985a. A classification of the Amphibia and Reptilia of Australia. Australian Journal of Herpetology, Supplementary Series, (1):1-61.
Wells, R. W. and Wellington, C. R. 1985b. A synopsis of the Amphibia and Reptilia of Australia. Australian Journal of Herpetology, Supplementary Series, (1):62-64.
Wilson, S. K. and Knowles, D. G. 1988. Australia's Reptiles: A Photographic Reference to the Terrestrial Reptiles of Australia. Collins Publishers, Sydney, NSW, Australia. 447 pp.
Worrell, E. 1972. Dangerous snakes of Australia and New Guinea, Angus and Robertson, Sydney, Australia. 65 pp.
* Papers can be downloaded from the world wide web at http://www.smuggled.com/pap1.htm
![]()
Raymond Hoser has been an active herpetologist for about 30 years and published over 150 papers in journals worldwide. He has written nine books including the definitive works "Australian Reptiles and Frogs", "Endangered Animals of Australia" and the controversial best sellers "Smuggled - The Underground Trade in Australia's Wildlife", "Smuggled-2", "Victoria Police Corruption" and "Victoria Police Corruption - 2"
![]()
Click here to download and view a 3.3 mb mpg (video) file of two mating Sydney Death Adders.![]()
Click here to download and view a 4.4 mb mpg (video) file of two mating Death Adders (A. antarcticus X A. cummingi).![]()
Click here to download and view a different (and more graphic) 7.2 mb mpg (video) file of two mating Death Adders (A. antarcticus male (different to above) X A. cummingi female).![]()
Australia's best snake handling courses.
Snakebusters: Australia's best reptile shows.
Non-urgent inquiries may be made via the e-mail address on the snakebusters bookings page at:
http://www.snakebusters.com.au/sbsboo1.htm
For urgent inquiries and bookings, phone: (Melbourne, Victoria, Australia):
(03) 9812 3322 or 0412 777 211

 To download the 5 mb pdf (Acrobat) file of the
exact article as it appeared in the journal Monitor (incl. colour photos
of type specimens) - click here - it will take about 30-40 minutes to download.
To download the 5 mb pdf (Acrobat) file of the
exact article as it appeared in the journal Monitor (incl. colour photos
of type specimens) - click here - it will take about 30-40 minutes to download.